What is geographic atrophy?

The increasing burden of age-related macular degeneration (AMD)
Geographic atrophy is an advanced form of AMD1–3 that causes progressive, irreversible degeneration of the macula.3–6
The global prevalence of AMD is growing at an exponential rate.2

AMD is a leading cause of permanent vision loss8
In 2020, the leading causes were cataract, followed by glaucoma, undercorrected refractive error, AMD and diabetic retinopathy.8
Causes of permanent vision loss in those aged 50+
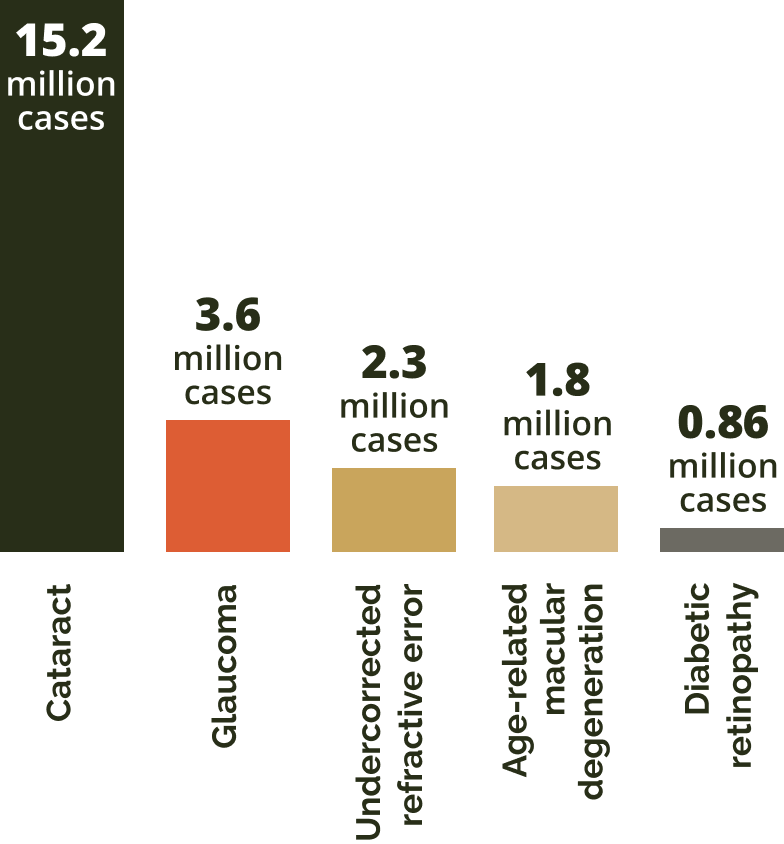
Adapted from GBD 2019.8
Among these global cases of AMD, the number of patients diagnosed with geographic atrophy cases is expected to almost quadruple – rising from 5 million to more than 18 million by 2040.2,3
Causes of permanent vision loss in those aged 50+
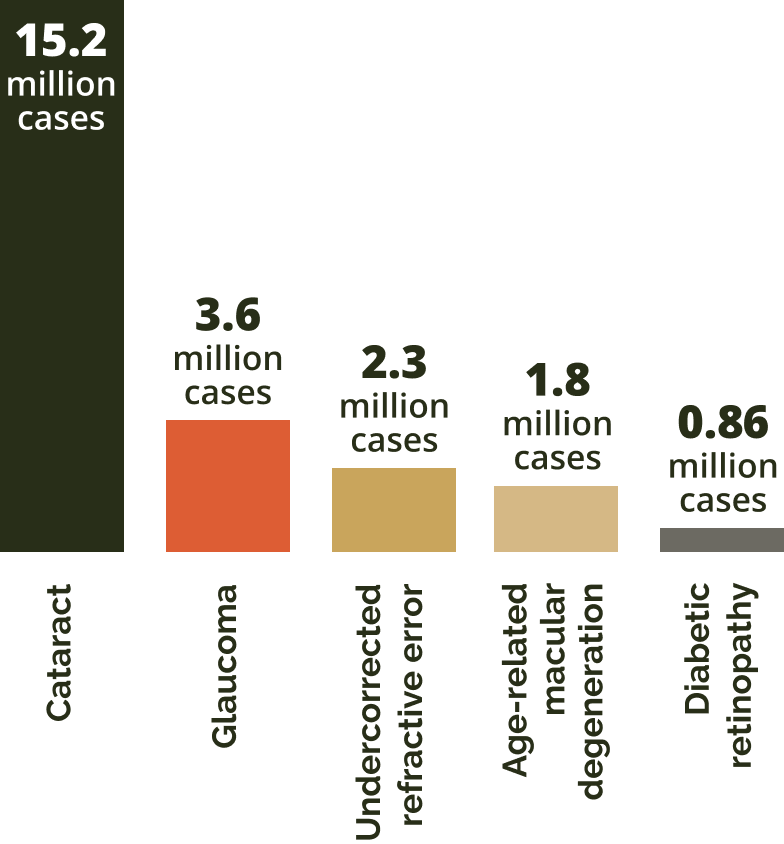
Adapted from GBD 2019.8
In 2020, the leading causes were cataract, followed by glaucoma, undercorrected refractive error, AMD and diabetic retinopathy.8
Among these global cases of AMD, the number of patients diagnosed with geographic atrophy cases is expected to almost quadruple – rising from 5 million to more than 18 million by 2040.2,3
Forms of advanced AMD
Advanced AMD has two forms: geographic atrophy and choroidal neovascularisation (wet AMD).9
Advanced AMD is less prevalent than the early and intermediate stages,10 however, it is often highly symptomatic and can lead to permanent vision loss.7

Geographic atrophy (GA) and wet AMD are considered different manifestations of advanced AMD but can occur simultaneously in the same eye. Patients with GA can naturally develop wet AMD and vice versa.9
Causes of geographic atrophy
AMD is a complex, multifactorial disease and GA pathogenesis encompasses a complex interaction of genetic, physiological and environmental factors.11–14
Genetics
- Drusen formation
- Formation of reactive oxygen species
- Inflammation
- Immune response, including complement
Physiology
- Age is the greatest risk factor for GA
- Certain types of dyslipidaemias
Environment
- Sunlight, smoking and diet
- High alcohol intake
Assessing AMD severity and risk of progression
The Beckman classification system recognises an increasing risk of developing advanced AMD.15 This system only requires a clinical examination or colour fundus image to observe and analyse retinal abnormalities.16
Beckman clinical classification of AMD
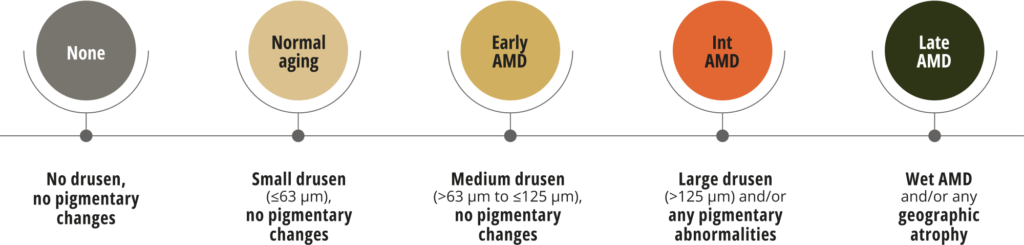

Adapted from Ferris III et al. 2013.15
Classification is based on assessment of fundus lesions within two disc diameters of the fovea in people over 55. The presence of large drusen (>125 μm) and/or pigmentary abnormalities indicate intermediate AMD with a high risk of progression to advanced AMD.15
Assessing your patients with the Beckman scale avoids ambiguity as to the staging of AMD and supports consistent classification.16
Diagnosis of geographic atrophy
Retinal imaging techniques are used to identify, diagnose and monitor all stages of AMD, including GA.17
When diagnosing and monitoring AMD, the following features can be detected in the retina:18
- Presence of drusen
- A sharply demarcated area in the macular region with an atrophic retina, lacking pigmentation
- Visible underlying choroidal blood vessels
Using multimodal imaging to assess GA severity and risk of progression16,19
Because of poor correlation between best-corrected visual acuity and geographic atrophy lesions or progression, a multimodal imaging approach is advised to better follow up GA patients:19
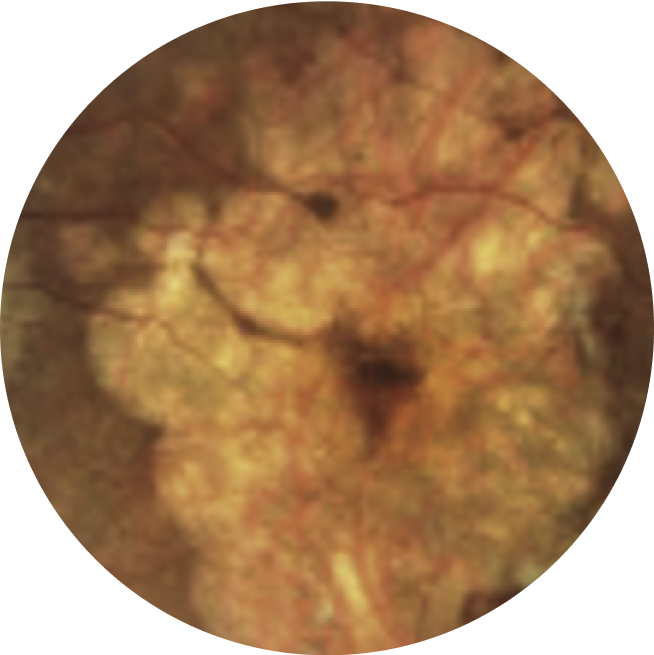
Colour fundus photography has traditionally documented GA lesions as an abrupt transition of fundus pigmentation resulting from the atrophy of retinal pigment epithelium (RPE) cells19
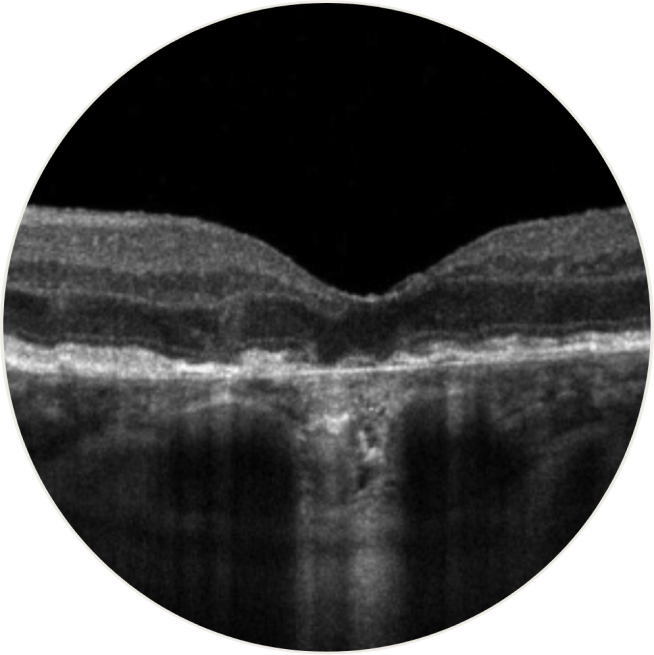
Spectral-domain optical coherence tomography provides detailed, in vivo images of the macula. Scans of GA reveal RPE thinning, depression of inner retinal layers as the outer layers are lost, and increase in visibility of Bruch’s complexes. Atrophic areas show clumps of hyperreflective material, segmented plaques of the outer band with variable reflectivity, and thickened outer hyperreflective bands. Dehydration and calcification of drusen are also seen on OCT20
- OCT has revealed near histological details of what appear to be the first signs of cell loss and the beginning of atrophy in eyes with AMD that only have drusen and pigmentary abnormalities, before clinically apparent signs of GA16
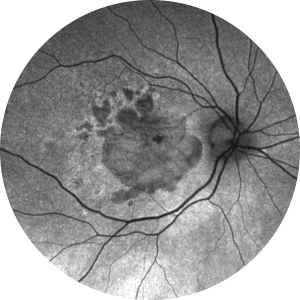
Fundus autofluorescence is useful for monitoring progressive GA enlargement, allowing a more reproducible measure of atrophic areas and a better lesion boundary discrimination.19 Loss of RPE autofluorescence indicates damage or death of photoreceptors and/or RPE cells19
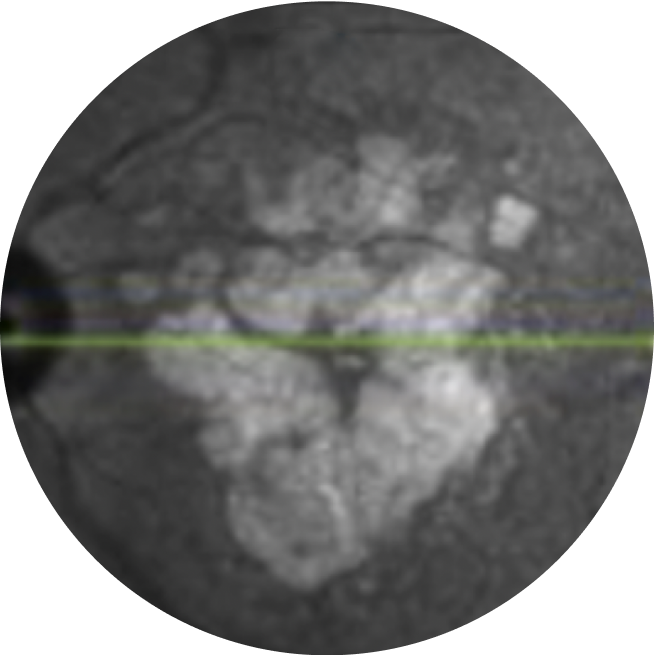
Near-infrared reflectance combined with fundus autofluorescence can indicate foveal sparing, which is important for patients’ visual acuity21
The CAM group (Classification of Atrophy Meetings) recommend OCT as the reference standard to diagnose and stage geographic atrophy.22
Fundus autofluorescence, near-infrared reflectance and colour imaging can be used to help confirm a GA diagnosis.22
Join us on our journey in geographic atrophy
Be the first to receive the latest geographic atrophy news
Thank you for submitting your details.
Please check your inbox for confirmation


Progression in geographic atrophy
Geographic atrophy is progressive and irreversible, eventually leading to permanent vision loss.3–6

Patients can lose more than their sight to geographic atrophy
They may have difficulty with independence, relationships, and everyday activities.23–25
Complement overactivation in geographic atrophy
Overactivation of the complement system can be detrimental and is associated with several serious diseases, including geographic atrophy.26
References
- Biarnés M, et al. Optom Vis Sci. 2011;88(7):881–889.
- Wong WL, et al. Lancet Glob Health. 2014;2(2):e106–e116 and supplementary appendix.
- Fleckenstein M, et al. Ophthalmology. 2018;125(3):369–390.
- Boyer DS, et al. Retina. 2017;37(5):819–835.
- Lindblad AS, et al. Arch Ophthalmol. 2009;127(9):1168–1174.
- Holz FG, et al. Ophthalmology. 2014;121(5):1079–1091.
- Stahl A. Dtsch Arztebl Int. 2020;117(29-30):513–520.
- GBD 2019 Blindness and Vision Impairment Collaborators. Lancet Glob Health. 2021;9(2):e144–e160.
- Kaszubski P, et al. Ophthalmic Res. 2016;55(4):185–193.
- Li JQ, et al. Br J Ophthalmol. 2020;104(8):1077–1084.
- Buschini E, et al. Clin Ophthalmol. 2015;9:563–574.
- AREDS Research Group. Ophthalmology. 2000;107(12):2224–2232.
- Fritsche LG, et al. Nat Genet. 2016;48(2):134–143.
- Chong EWT, et al. Am J Ophthalmol. 2008;145(4):707–715.
- Ferris FL III, et al. Ophthalmology. 2013;120(4):844–851.
- Guymer R. Rethinking our AMD nomenclature. Retina Today, 2022;32–34.
- American Academy of Ophthalmology. What is the difference between direct and indirect ophthalmoscopy. 2016. Available at: https://www.aao.org/eye-health/ask-ophthalmologist-q/what-is-difference-between-direct-indirect-ophthal (Accessed May 2023).
- EyeWiki. Geographic atrophy. 2021. Available at: https://eyewiki.aao.org/Geographic_Atrophy (Accessed May 2023).
- Sacconi R, et al. Ophthalmol Ther. 2017;6(1):69–77.
- Elsharkawy M, et al. Diagnostics (Basel). 2021;11(12):2313.
- Lindner M, et al. Invest Ophthalmol Vis Sci. 2017;58(6):BIO61–BIO67.
- Sadda SR, et al. Ophthalmology. 2018;125(4):537–548.
- Sivaprasad S, et al. Ophthalmol Ther. 2019;8(1):115–124.
- Jones D, et al. Invest Ophthalmol Vis Sci. 2022;63(7):A0145.
- Apellis & The Harris Poll. 2022. Geographic Atrophy Insights Survey (GAINS).
- Liao DS, et al. Ophthalmology. 2020;127(2):186–195.
EU-GA-2300007 May 2023